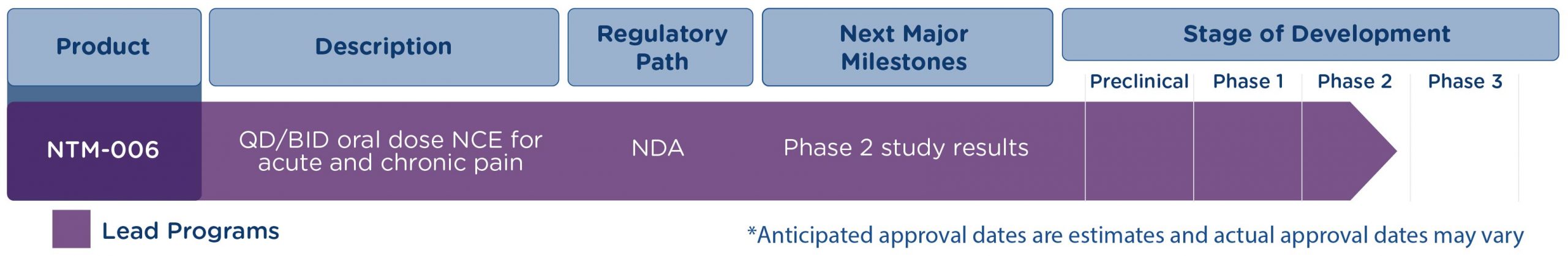
NTM-006
NCE: New Chemical Entity, NDA: New Drug Application
NTM-006 is a New Chemical Entity with a Novel Mechanism of Action
- Non-opioid, non-NSAID, non-gabapentinoid new chemical entity (NCE) with a novel mechanism of action (MOA) for once- or twice-daily, oral administration for the treatment of acute and chronic pain.
- In-licensed from J&J which developed the asset and conducted a phase 2a single-dose study demonstrating superior analgesic efficacy vs acetaminophen over 24 hours.
- Preclinical evidence suggests NTM-006 exerts its analgesic activity via Adenosine A3 Receptor Modulation (ARM).
- In development for acute and chronic pain with the potential to offer significant benefits as monotherapy or as part of a multimodal regimen based on:
- Convenient once- or twice-daily, oral regimen.
- Non-opioid that is non-scheduled, and devoid of opioid induced side-effects, respiratory depression and abuse potential.
- Non-NSAID without the NSAID boxed warning or potentially serious NSAID related gastrointestinal, cardiovascular or bleeding side effects.
- Non-gabapentinoid with more predictable efficacy and superior tolerability.
Computer modeling shows NTM-006 molecule fits allosteric binding sites of adenosine A3 receptors, consistent with MoA of A3 receptor modulation.
If approved, NTM-006 will provide patients, professionals and payers with a new, effective, non-opioid analgesic, with a novel MOA, and without the side-effects, risks, and liabilities of opioids, or the disadvantages of NSAIDs and gabapentinoids.
Disclaimer: NTM-006 is an investigational new drug candidate and is not approved for any indication in any market.