Pipeline
Pipeline
NCE: New Chemical Entity, NDA: New Drug Application, PMDA: Pharmaceuticals and Medical Devices Agency (Japan)
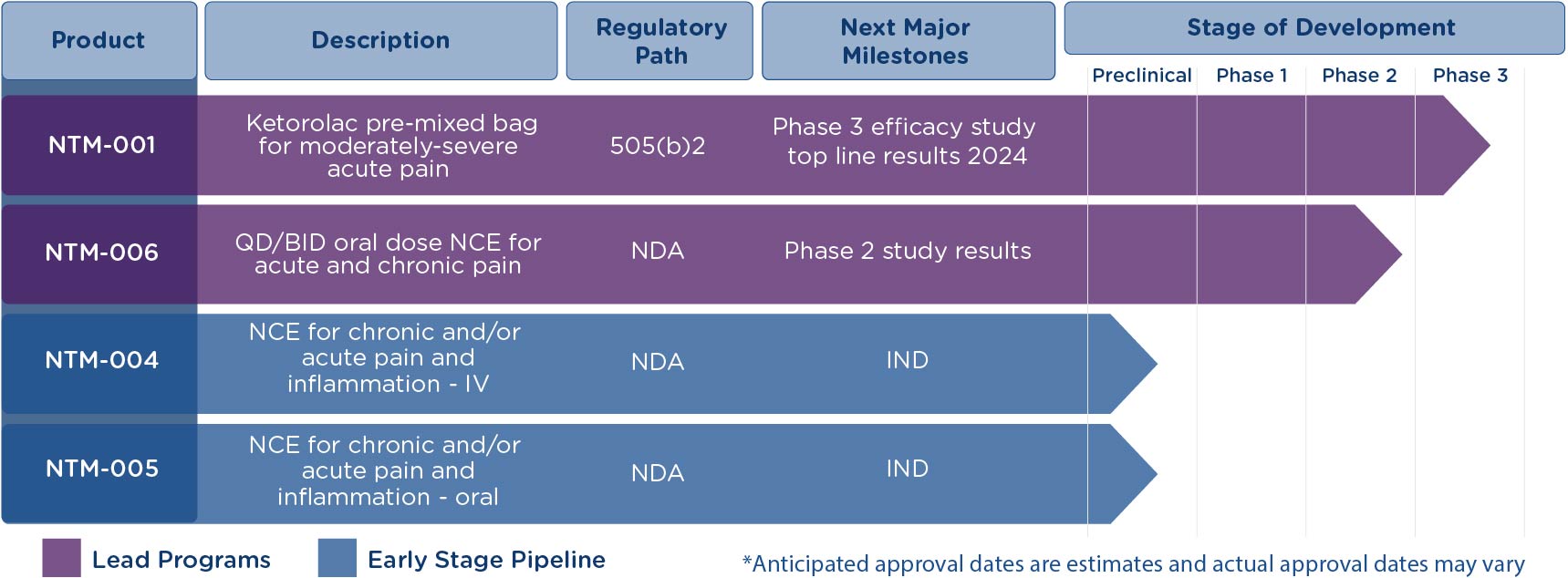
Neumentum’s pipeline aims to address shortfalls of current pain management treatments by developing and commercializing effective and safe, non-opioid options without the risks of abuse, misuse and diversion seen with opioids, nor the opioid-induced side effects, including potentially life-threatening respiratory depression.
NTM-006
A new chemical entity with a novel mechanism of action (MoA). In development for acute and chronic pain with the potential to offer significant benefits as monotherapy or as part of a multimodal regimen.
NTM-001
A novel formulation of ketorolac in a pre-mixed bag (PMB) designed to deliver 24 hours of opioid level analgesia, reduce the potential analgesic gaps associated with bolus ketorolac, reduce or eliminate the need for postoperative opioids, and shorten the length of hospital stay.
Early-Stage Pipeline
Including a short infusion of ketorolac PMB for the treatment of acute pain, and two non-opioid NCE analgesic compounds with the potential to provide powerful efficacy and without the liver toxicity associated with APAP.
Disclaimer: These are investigational new drug candidates and are not approved for any indication in any market