Early Stage
Pipeline
Early Stage
Pipeline
NCE: New Chemical Entity, NDA: New Drug Application, PMDA: Pharmaceuticals and Medical Devices Agency (Japan)
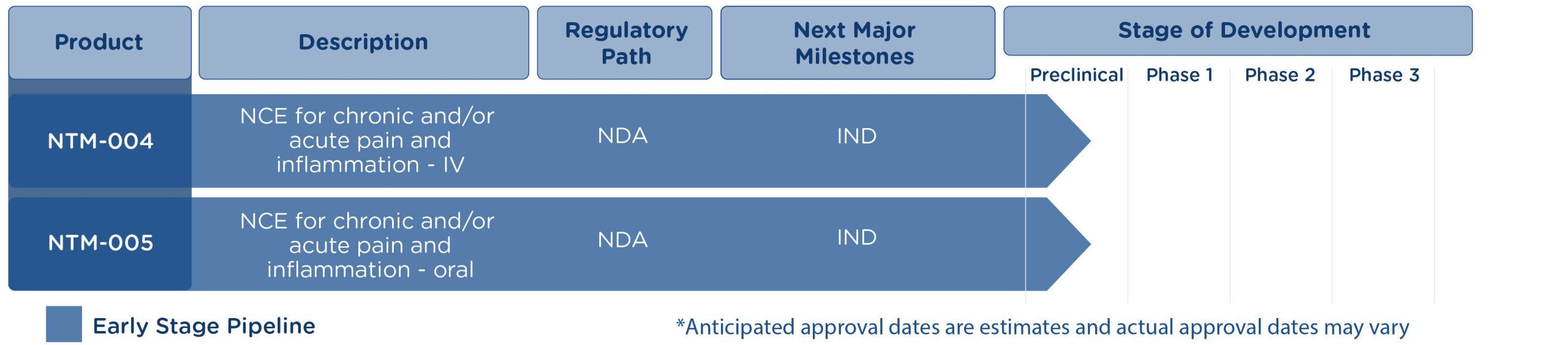
Earlier stage products include a short infusion of the novel formulation of ketorolac in a premixed bag (PMB) and two non-opioid NCE analgesic compounds with the potential to provide powerful efficacy and without the liver toxicity associated with acetaminophen (APAP). Liver toxicity can be life-threatening and APAP overdose is the leading cause of acute liver failure in the U.S.1
APAP is an active ingredient in over 600 prescribed and over-the-counter products, including Percocet, Vicodin, Tylenol and Excedrin.
Neumentum’s products have the potential to replace APAP in the prescription and OTC markets, with superior efficacy and reduced risk of adverse effects such as liver failure.
- Larson AM et al. Hepatology. 2005;42(6):1364-72
Disclaimer: These are investigational new drug candidates and are not approved for any indication in any market.